Mark Dominus (陶敏修)
mjd@pobox.com

Archive:
| 2025: | JFMAMJ |
| JAS | |
| 2024: | JFMAMJ |
| JASOND | |
| 2023: | JFMAMJ |
| JASOND | |
| 2022: | JFMAMJ |
| JASOND | |
| 2021: | JFMAMJ |
| JASOND | |
| 2020: | JFMAMJ |
| JASOND | |
| 2019: | JFMAMJ |
| JASOND | |
| 2018: | JFMAMJ |
| JASOND | |
| 2017: | JFMAMJ |
| JASOND | |
| 2016: | JFMAMJ |
| JASOND | |
| 2015: | JFMAMJ |
| JASOND | |
| 2014: | JFMAMJ |
| JASOND | |
| 2013: | JFMAMJ |
| JASOND | |
| 2012: | JFMAMJ |
| JASOND | |
| 2011: | JFMAMJ |
| JASOND | |
| 2010: | JFMAMJ |
| JASOND | |
| 2009: | JFMAMJ |
| JASOND | |
| 2008: | JFMAMJ |
| JASOND | |
| 2007: | JFMAMJ |
| JASOND | |
| 2006: | JFMAMJ |
| JASOND | |
| 2005: | OND |
Subtopics:
| Mathematics | 245 |
| Programming | 99 |
| Language | 95 |
| Miscellaneous | 75 |
| Book | 50 |
| Tech | 49 |
| Etymology | 35 |
| Haskell | 33 |
| Oops | 30 |
| Unix | 27 |
| Cosmic Call | 25 |
| Math SE | 25 |
| Law | 22 |
| Physics | 21 |
| Perl | 17 |
| Biology | 16 |
| Brain | 15 |
| Calendar | 15 |
| Food | 15 |
Comments disabled
Wed, 09 Sep 2015
A message to the aliens, part 7/23 (mass)
Earlier articles: Introduction Common features Page 1 (numerals) Page 2 (arithmetic) Page 3 (exponents) Page 4 (algebra) Page 5 (geometry) Page 6 (chemistry)
This is page 7 of the Cosmic Call message. An explanation follows.

The 10 digits are:
 0 |  1 | 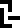 2 | 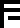 3 | 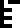 4 | 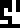 5 | 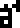 6 | 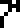 7 | 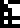 8 | 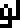 9 |
Page 7 discusses mass and defines the kilogram.
The top section of the page continues the table of chemical elements
from the previous page, giving the number of protons and neutrons.
For example, gold 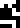 is described as having 79 protons
is described as having 79 protons 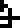 and 117 neutrons
and 117 neutrons
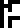 .
.
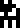 Sulfur | 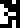 Zinc | 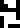 Argon | 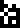 Silver | 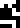 Gold |  Uranium | 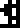 Copernicium |
Copernicium, element 112, had been discovered but not named at the time the document was written.
There is a major error here.
Uranium
 is given as having 92 protons and 116
neutrons. There is no such substance. It should have said 146
neutrons.
is given as having 92 protons and 116
neutrons. There is no such substance. It should have said 146
neutrons.

I sometimes imagine the aliens, having received the message, come to visit us. “We weren't going to bother,” they say, “but we had to know about the uranium-208.” And then we will have to tell them that we messed up. Ouch. (It could be an error for lead-208 or bismuth-208 instead; one can't be sure because the glyph does not appear elsewhere in the document.)
I'd been planning to write that paragraph about uranium-208 for more
than ten years, but it wasn't until just now that I realized there is
a much more serious mistake two lines down, so that the uranium is no longer
the most serious error that I know of in the entire document. The
line after the table of elements says that the mass of a carbon atom is the mass of six
protons plus the mass of six neutrons plus “energy”, 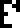 , by which I think
they mean the binding energy in the nucleus. This is the first
appearance of the glyph for energy, which will recur later. And then
the following line commits a really horrible boner, one that has the
potential to spoil the whole message.
, by which I think
they mean the binding energy in the nucleus. This is the first
appearance of the glyph for energy, which will recur later. And then
the following line commits a really horrible boner, one that has the
potential to spoil the whole message.
With the mass of the carbon nucleus pinned down, the authors want to
define the kilogram 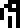 : the document
says that 12 kilograms is the mass of !!6022137\cdot 10^{19} !!
carbon-12 atoms. That !!6022137\cdot 10^{19} !! is Avogadro's number.
Except it's not. Avogadro's number is usually given as
!!6.022137\cdot 10^{23} !!, and this number is 100 times that big.
But it should be 1000 times that big.
: the document
says that 12 kilograms is the mass of !!6022137\cdot 10^{19} !!
carbon-12 atoms. That !!6022137\cdot 10^{19} !! is Avogadro's number.
Except it's not. Avogadro's number is usually given as
!!6.022137\cdot 10^{23} !!, and this number is 100 times that big.
But it should be 1000 times that big.
Normally, one would say that !!6.022137\cdot 10^{23} !! carbon atoms mass 12 grams, but there are two confusing factors here. One is that the authors have written !!6022137!! instead of !!6.022137!! and the other is that they are defining 12 kilograms instead of 12 grams. But it should be that !!6.022137\cdot 10^{23} = 6022137\cdot 10^{17}!! atoms is 12 grams so that !!6022137\cdot 10^{20}!! atoms is 12 kilograms, and the number written is instead !!6022137 10^{19}!! atoms, making the kilogram 90% smaller than it should have been.

It's possible that the aliens can figure this out, because it is detectably inconsistent with the following statements about the masses of the fundamental particles in kilograms. But it may not be clear to the recipients which of the two definitions of the kilogram is the correct one. Especially given the—I really hate to report this—the typo in the second statement.
The three following lines give the masses of the proton, neutron, and electron in kilograms. These are all more or less correct (although the book values have changed since the message were written) and I think the value for the neutron has a typo; it says !!1.6739286\cdot 10^{{}^-34}!! kg but it probably should have been !!1.67{\mathit 4}9286\cdot 10^{{}^-34}!! kg which would agree with the current book value of !!1.674927351\cdot 10^{{}^-34}!! kg.

Since we're going over the errors on this page, here is yet another
oddity. The number of neutrons in a gold atom
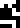 is given at the top of
the page as 117. Unlike uranium-208., the isotope gold-196 actually exists. But it is radioactive,
breaking down into platinum or mercury after about a week. One would
expect the listing to be for gold-197 instead, which is the only
stable isotope and so is the only isotope occurring in naturally-found
gold. (Thanks to Peter Annema for bringing this to my attention.)
A similar oddity occurs in the listing for zinc
is given at the top of
the page as 117. Unlike uranium-208., the isotope gold-196 actually exists. But it is radioactive,
breaking down into platinum or mercury after about a week. One would
expect the listing to be for gold-197 instead, which is the only
stable isotope and so is the only isotope occurring in naturally-found
gold. (Thanks to Peter Annema for bringing this to my attention.)
A similar oddity occurs in the listing for zinc
 :
zinc-65 is given instead of the stable zinc-64 or zinc-66.
The
other isotopes listed here (sulfur-32, argon-40, silver-107) are more
plausible.
:
zinc-65 is given instead of the stable zinc-64 or zinc-66.
The
other isotopes listed here (sulfur-32, argon-40, silver-107) are more
plausible.
[Other articles in category /aliens/dd] permanent link



